Presentation Text for Cleanroom Mechanical Systems Project for COVID-19 Vaccine Production
Introduction:
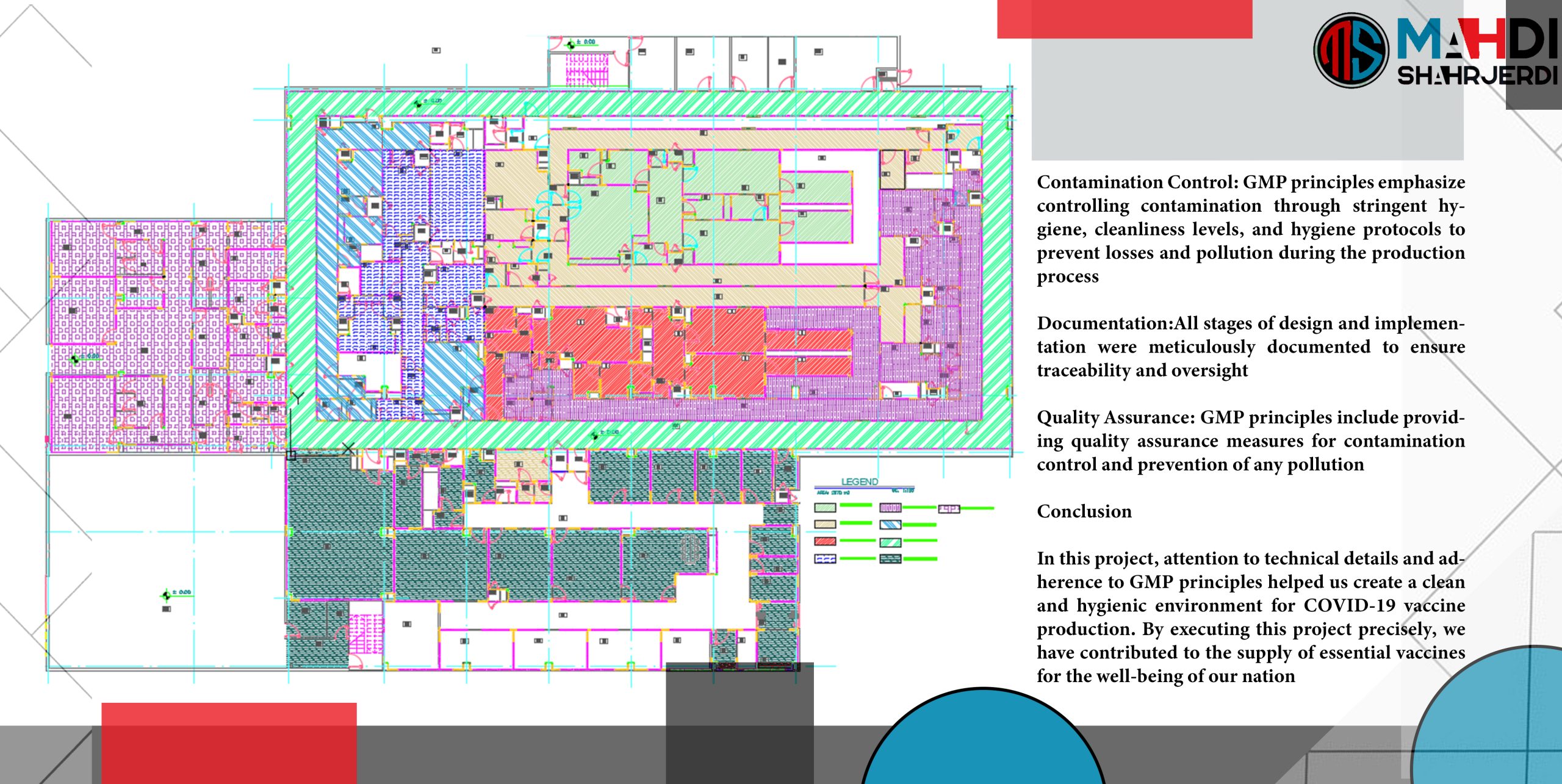
Greetings and respect. Here, we provide you with the presentation text for the Cleanroom Mechanical Systems Project for COVID-19 vaccine production. This project is dedicated to designing and implementing a hygienic and completely secure environment for the production of vital vaccines for our society.
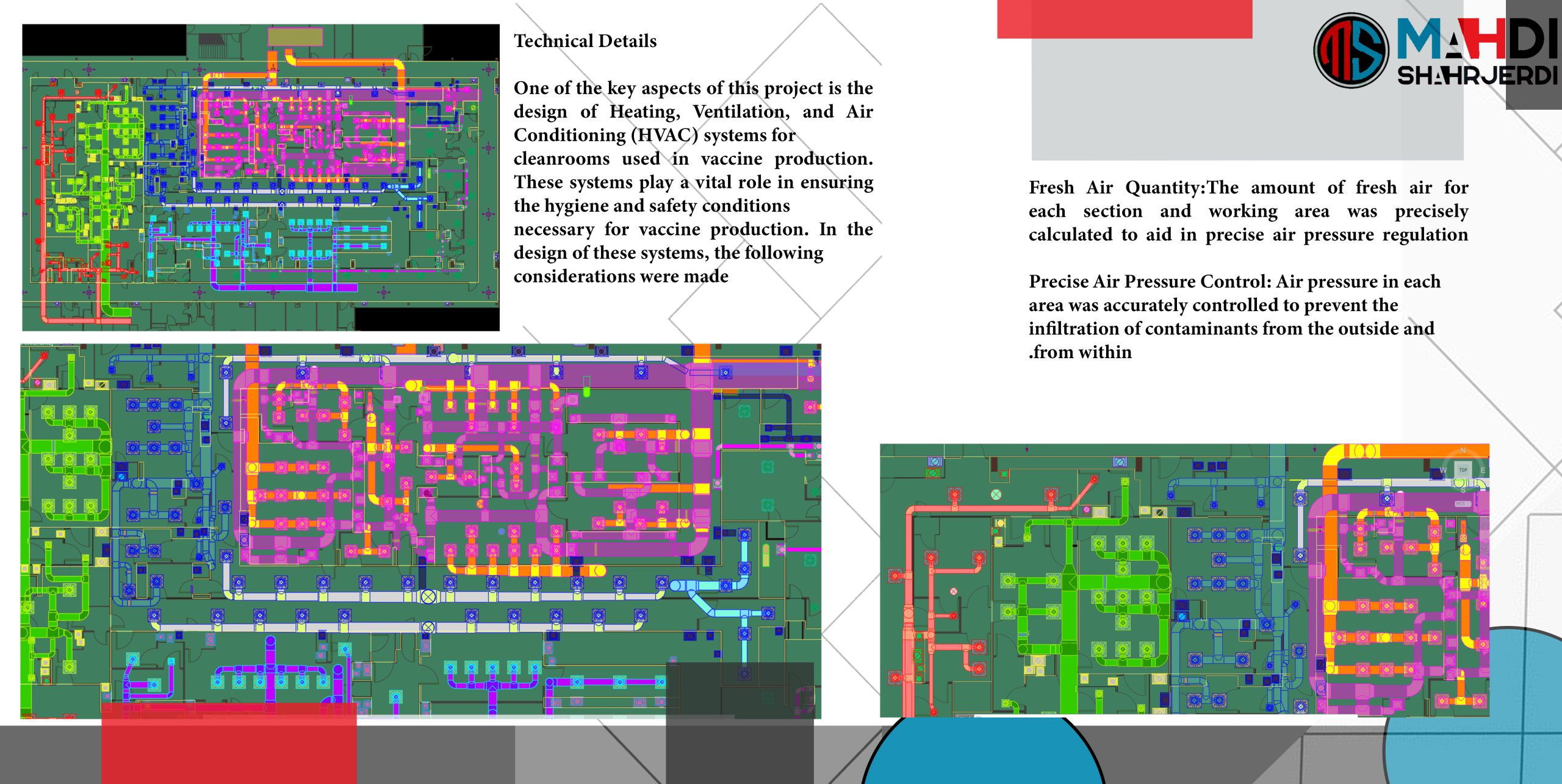
Technical Details:
One of the key aspects of this project is the design of Heating, Ventilation, and Air Conditioning (HVAC) systems for cleanrooms used in vaccine production. These systems play a vital role in ensuring the hygiene and safety conditions necessary for vaccine production. In the design of these systems, the following considerations were made