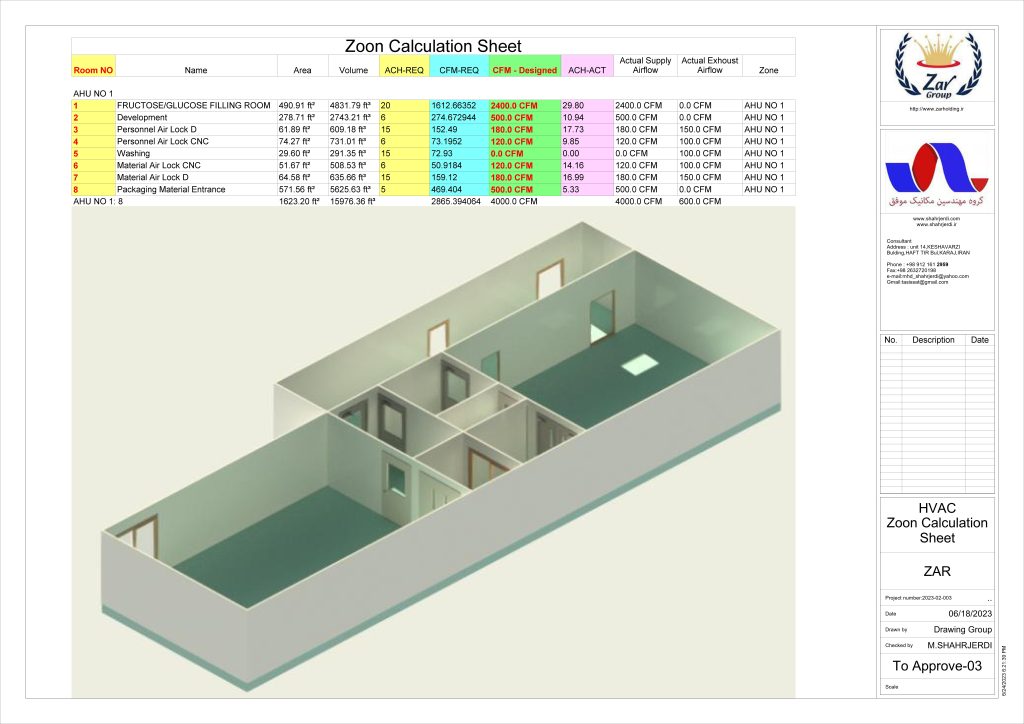
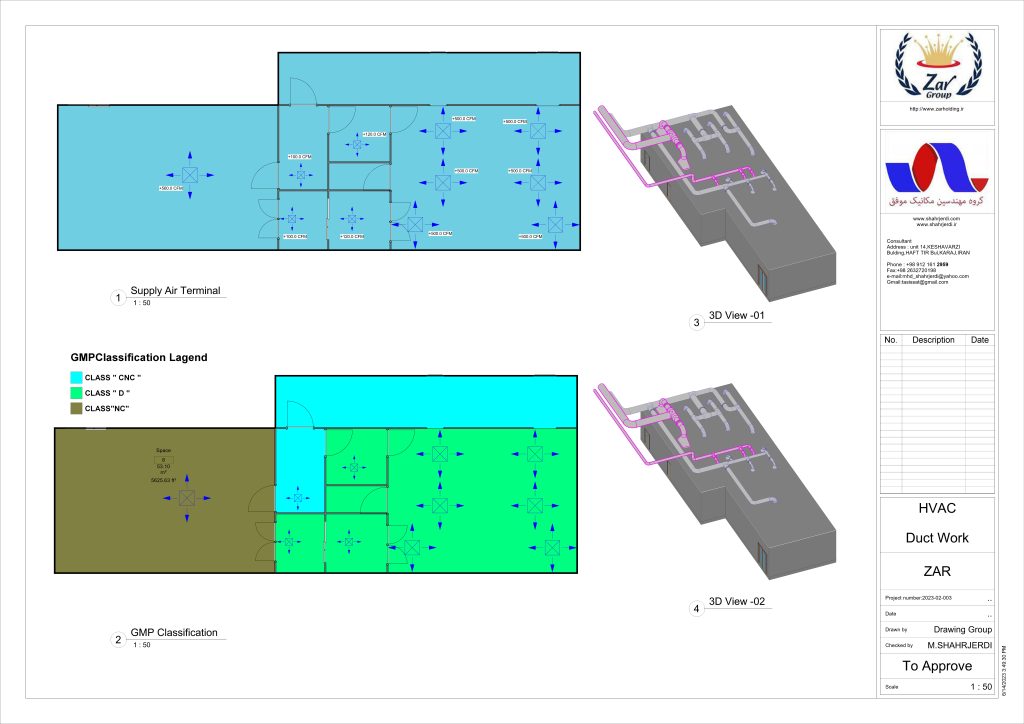
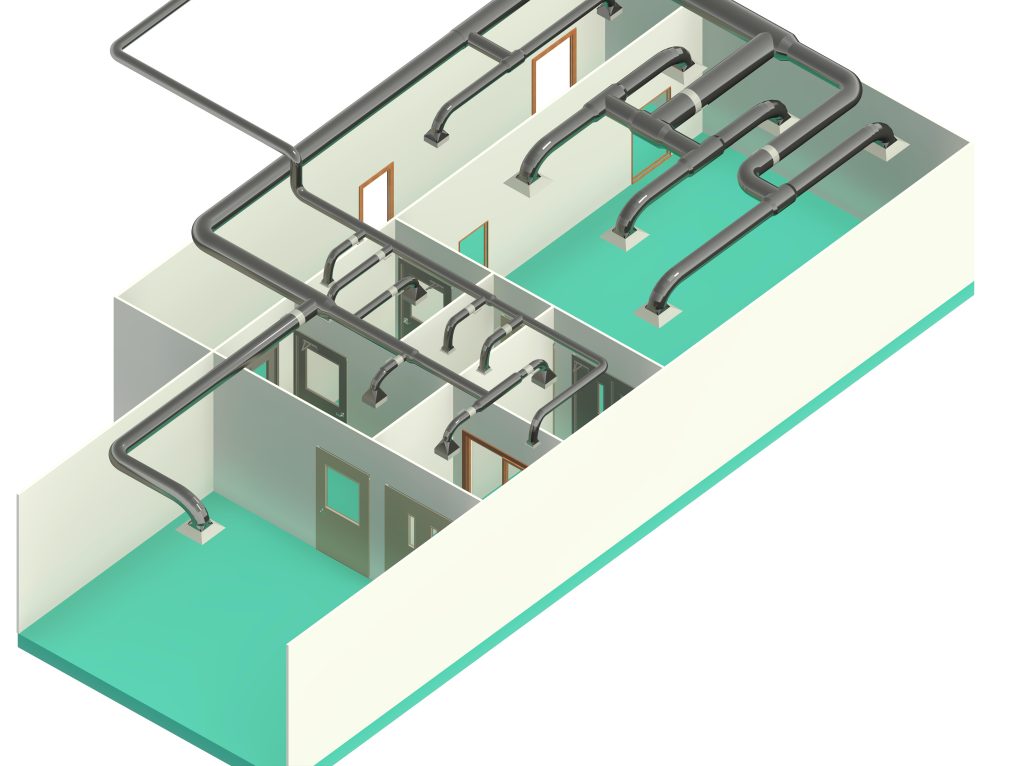
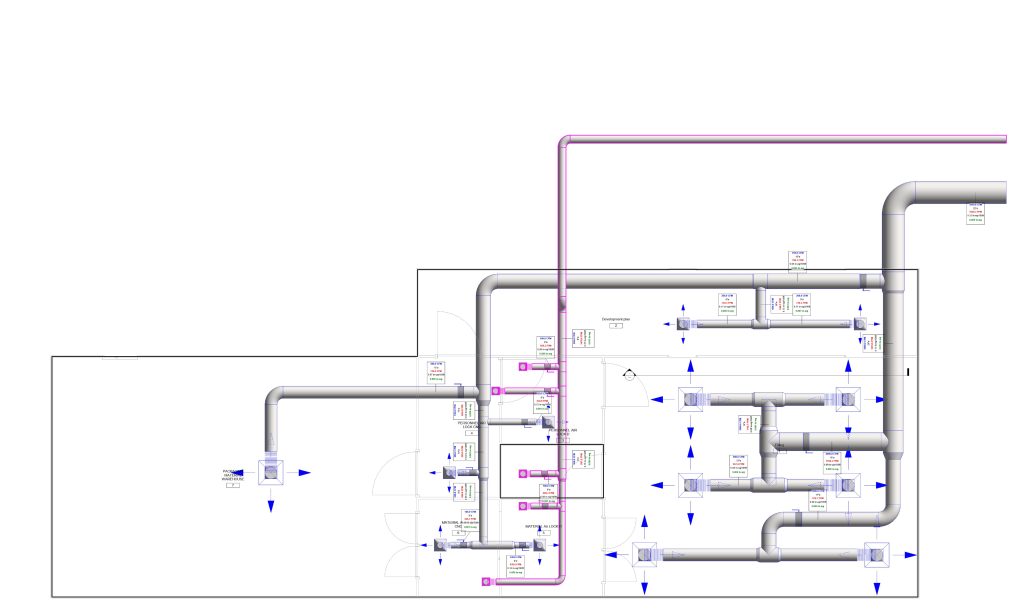
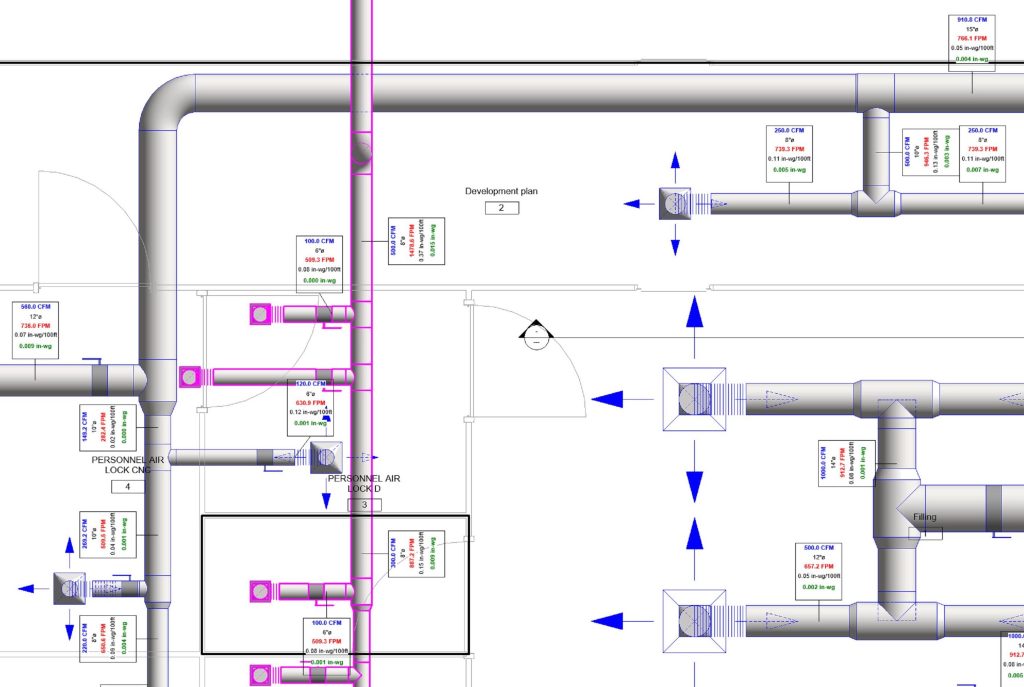
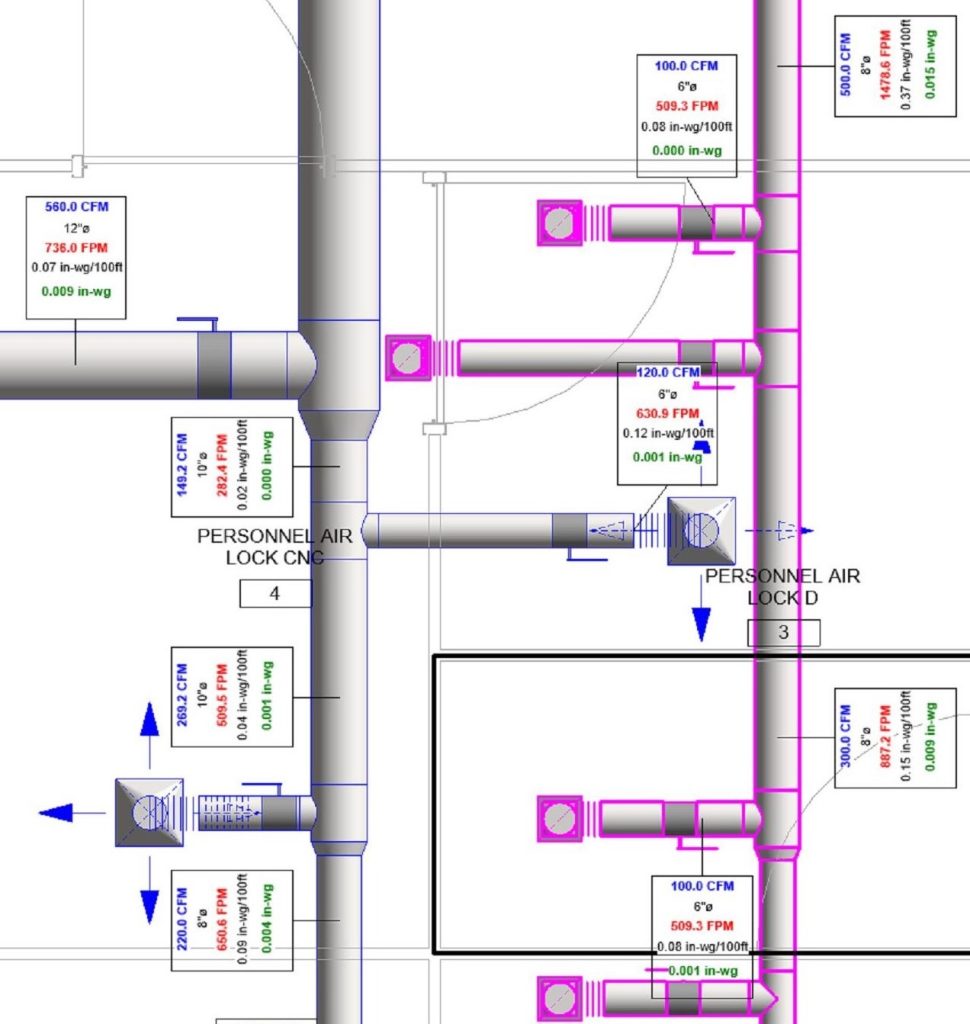
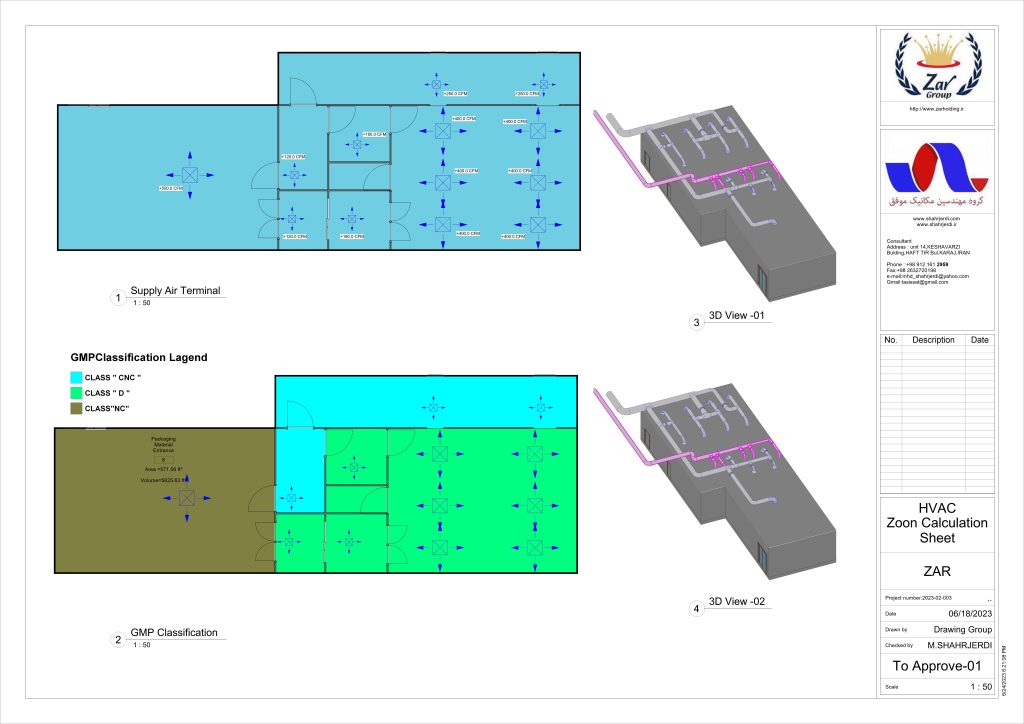
In conclusion, I proudly declare that Project Clean Room Sugar No. 1, as a prime example of complete compliance with GMP principles, plays a vital role in the realm of food and pharmaceutical industries in Iran. The efforts and expertise employed in this project, aimed at enhancing the quality and health of products and elevating levels of efficiency and effectiveness, are aligned with the main objectives of these industries. This project is a testament to our commitment to the health and well-being of consumers and the significant role we play in society